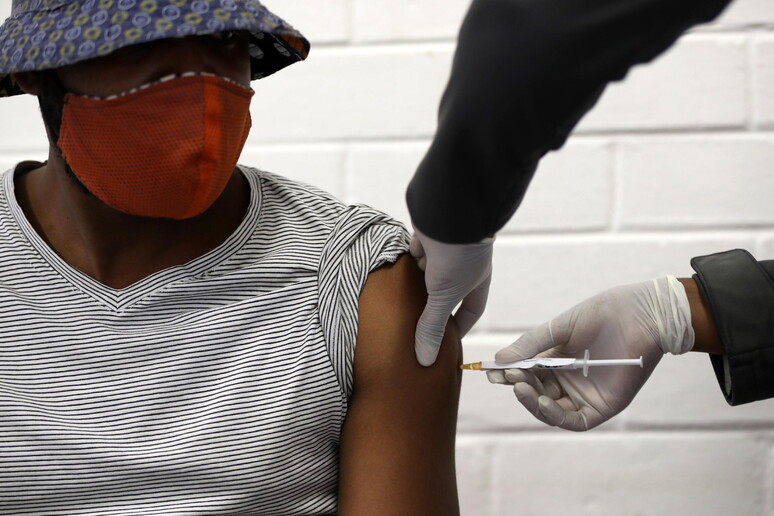
The ChAdOx1 anti-COVID vaccine,
developed by Oxford University's Jenner Institute with the
collaboration of Italy's Irbm company, "induced a strong immune
and anti-body response up to the 56th day of the ongoing
testing," The Lancet reported Monday. These are the preliminary
results of phase 1-2 of testing which involved 1,077 healthy
adults. "Further studies are necessary to confirm if the vaccine
effectively protects from COVID-19", the article said.
The article, published in the authoritative scientific journal,
highlights "promising initial results" relating to the ChAdOx1
vaccine, which is described as "safe" and "with few side
effects". The preliminary results have in fact shown that in the
sample of 1,077 healthy adult subjects involved in the testing,
the vaccine was able to trigger "strong responses" in the
production of anti-bodies and T immune cells up to day 56 of the
ongoing clinical testing. The responses, the Jenner Institute
researchers underscore, "may even be greater after a second
dose, according to a study on a sub-group of 10 participants".
The authors, however, urge caution, stressing that "further
clinical studies should be carried out on this vaccine
prototype". The present results, they point out, are in fact
focused on the immune response measured in the laboratory and
"further tests are necessary to confirm if the vaccine
effectively protects from COVID-19 infection".
"More time and patience is needed. But the first scientific
results of the Oxford University vaccine, whose viral vector is
made in Pomezia and will be placed in phials at Anagni, are
encouraging," said Health Minister Roberto Speranza. "Italy,
with Germany, France and the Netherlands," he added, "is in the
lead group for this testing. We are continuing to invest in
scientific research as a key to defeat the virus".
The positive results of the anti-COVID vaccine tests performed
in Oxford is "good news" for the World Health Organization (WHO)
too, according to the agency's health emergencies director
Michael Ryan, who conveyed to his colleagues at the British
university his "congratulations for the progress made". The fact
that the vaccine developed by the British university has
produced a strong immune response "is a positive result, but
there is still a long way to go," Ryan said. "Now we have to
move on to large-scale tests", he added.
ALL RIGHTS RESERVED © Copyright ANSA