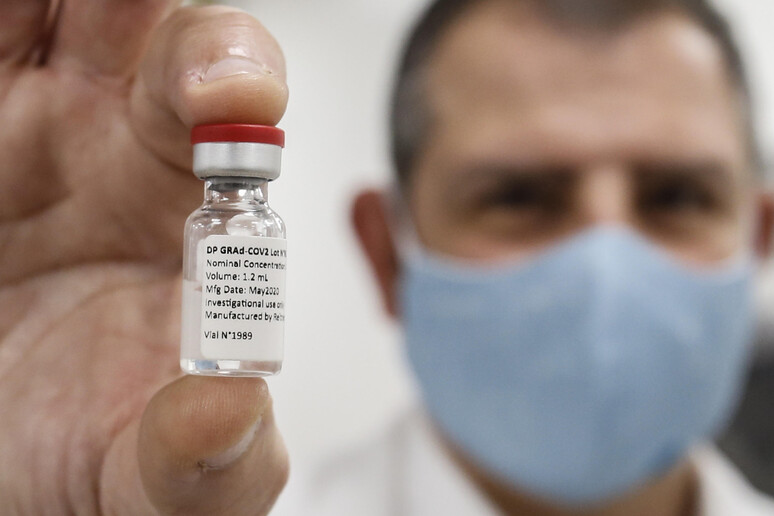
(ANSAmed) - ROME, JUL 12 - Italy's ReiThera vaccine against
COVID-19 showed an antibody response against the Spike protein
in over 93% of volunteers three weeks after the first dose,
reaching 99% after the second shot, the biotech company based in
Castel Romano, near Rome, said on Monday.
Five weeks after the first vaccination the level of
antibodies that connect the Spike protein and neutralize the
virus was comparable to that measured in a group of patients
recovering from the Covid infection who were used as a control
group, the company explained.
ReiThera went on to say that "the results after the first
five weeks from the start of the vaccination confirmed what was
already observed during Phase 1: the vaccine is well tolerated
in the first dose and even better tolerated after the second.
"Adverse events, for the most part of a small or moderate
entity and of short duration, are mainly referable to pain and
tension at the site of the injection, sense of fatigue, muscular
pain and headache", the company went on to say.
"No serious adverse events that could be connected to the
vaccine were registered", it said.
Preliminary data on security and immunity during the first
five weeks under consideration were examined at a joint meeting
of the Data Safety Monitoring Board, the independent committee
for the evaluation of security and the Steering Committee, the
scientific committee for the evaluation of efficacy.
The two committees expressed a positive opinion based on the
data aand recommended the prosecution of the clinical
development of the vaccine GRAd-COV2.
The study, which began on March 18 in 24 clinical centers
across Italy, was conducted on 917 volunteers.
A reported 25% of volunteers were over 65 and/or had
conditions associated with an increased risk of severe illness
in case of infection from SARS-CoV-2. (ANSAmed).
ALL RIGHTS RESERVED © Copyright ANSA